Susceptibility to sea lice involves responses by both the parasite and the host
Dr Laura M Braden

In Canada, the Atlantic salmon is the main species of finfish produced by the aquaculture industry, and accounts for ~ 65% of total production. Projections from Fisheries & Oceans Canada place production to reach 197,000 tonnes and $1.2 billion revenue by 2020. In the midst of this growth, it remains imperative for the industry to minimize potential negative ecological and environmental impacts. One of the largest contributors to economic losses in Atlantic salmon farming is disease caused by bacterial, viral, or parasitic pathogens. In addition to industrial losses, the potential for spread of infectious disease from net-pens to nearby populations of wild salmon has been a major impediment for growth of salmon aquaculture in Canada. One pathogen that receives considerable attention is the parasitic sea louse, Lepeophtheirus salmonis.
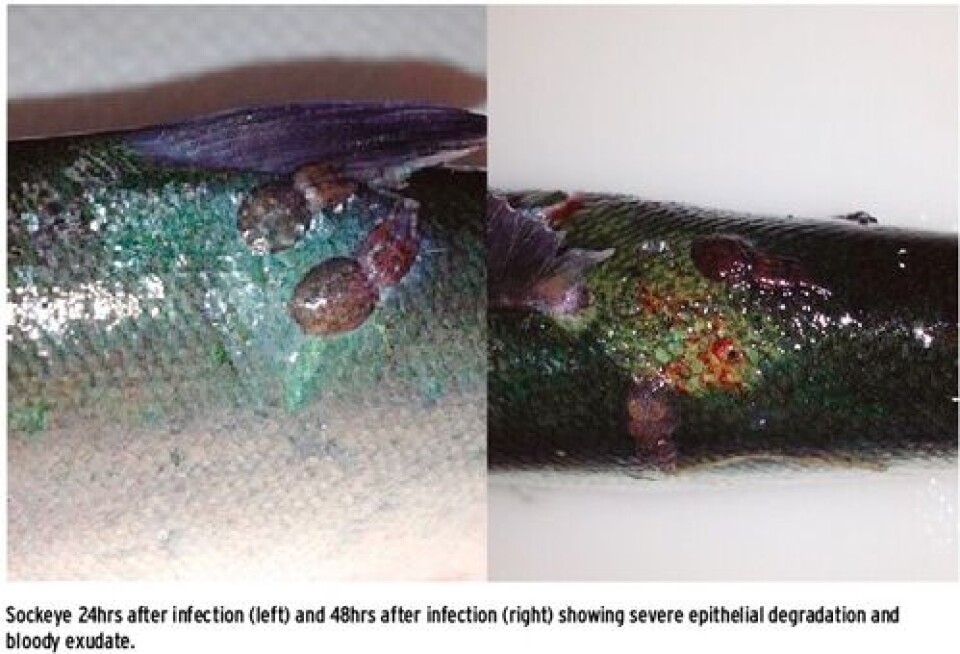
Management of L. salmonis on farmed salmon is heavily reliant on pesticide treatment although integrated pest management is becoming more frequently applied given the occurrence of reduced therapeutant efficacy from the development of pesticide resistance. Other methods of parasite management focus on augmenting or improving the immunological response to infection such as immune stimulants or selective breeding. A more thorough understanding of the defense mechanisms elicited by the sea louse at the skin attachment site and the extent to which local and systemic responses are integrated is imperative for further development of management strategies. My research has focused on characterizing the molecular mechanisms of salmonid resistance during infection with the salmon louse, Lepeophtheirus salmonis. By using a comparative approach, responses by both resistant (coho and pink salmon) and susceptible (Atlantic, chum and sockeye) salmon were investigated at the louse-attachment site. Furthermore, reciprocal responses by the louse were assessed as a function of attachment to these different host species. There is inadequate understanding of this host/parasite relationship, which is the limiting factor for the development of novel and sustainable strategies for parasite control. Development of parasiticide resistance on a global scale necessitates more permanent and sustainable approaches to sea lice treatment, and in the absence of an available vaccine, knowledge pertaining to resistance mechanisms will offer targets for selective breeding or immunostimulation to augment the natural immunity of susceptible species. Host-relationships involve responses by both the parasite and host. The primary interaction site in the louse/salmon relationship is the fish skin. With respect to the sea louse this site is characterized by an attack response such as attachment and feeding activities including secretion of bioactive molecules into the attachment site. In efforts to minimize pathological effects of sea louse infections there is a defense response by the salmon host such as inflammation, wound healing and hopefully, rejection of the parasite.
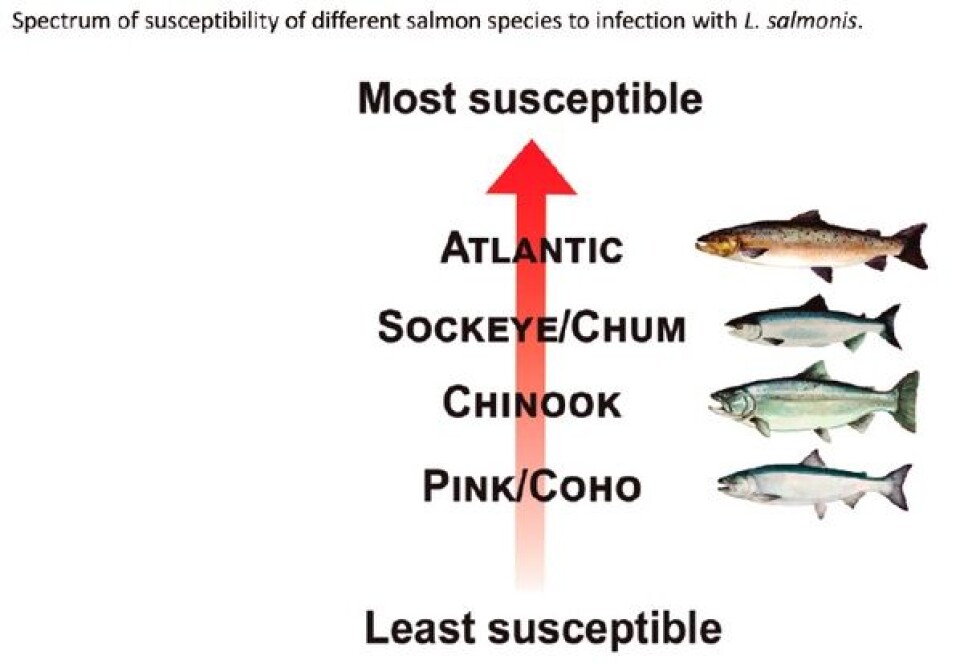
What is not yet clear is how the relationship of the defense response (inflammation) and the physiological factors of a particular host species (eg skin physiology), in combination to the attack responses of L. salmonis on that host (ie immunomodulation), contribute to successful attachment. Species-specific differences in the innate defense parameters at the mucosal/epithelial surface may be a critical determinant in successful infection by L. salmonis. Among species of Pacific salmon, juvenile pink and coho are the most resistant to L. salmonis. My research has shown that in the skin, this resistance is characterized by exaggerated expression of inflammatory mediators, acute-phase proteins, infiltration of cellular effectors and tissue remodelling enzymes. These data are supported by earlier transcriptomic studies that showed juvenile pink salmon respond to infection with L. salmonis by up-regulation of innate inflammatory genes, tissue remodelling, and iron regulatory pathways. The presence of regulatory immune pathways are typically associated with parasitic infections as they act to dampen the effects of sustained inflammation and permit wound healing and tissue remodelling. Until now, a TH2-type response was thought to be characteristic of susceptible species. However, the early and exaggerated expression of a major TH2-type cytokine in the skin of resistant coho salmon during infection with sea lice suggests that this pathway may be more pertinent than previously thought. This result suggests that not only is immediate inflammation an important anti-louse response, but that this inflammation needs to be controlled and switched to a regulated response permissive of wound healing and tissue remodelling. The skin/louse interface is also the site of feeding responses by the louse, including the secretion of immunomodulatory molecules. Earlier work found trypsin-like proteases in L. salmonis; however, the full characterization of the L. salmonis secretome was impeded by limited molecular resources. The availability of the entire genome of L. salmonis has provided opportunities for genomic, transcriptomic and now proteomic analysis of the sea louse. From the secretions of L. salmonis I have identified the presence of 52 proteins. Of these, 18 were proteases including cathepsins, carboxypeptidases, trypsins, collagenases, metallopeptidases and metalloproteinases, all known virulence factors in tick host/parasite relationships. This detailed inventory of proteins present in the secretome of L. salmonis represents an important component of the louse-salmon relationship and may be used to further explain observed variation in host susceptibility.
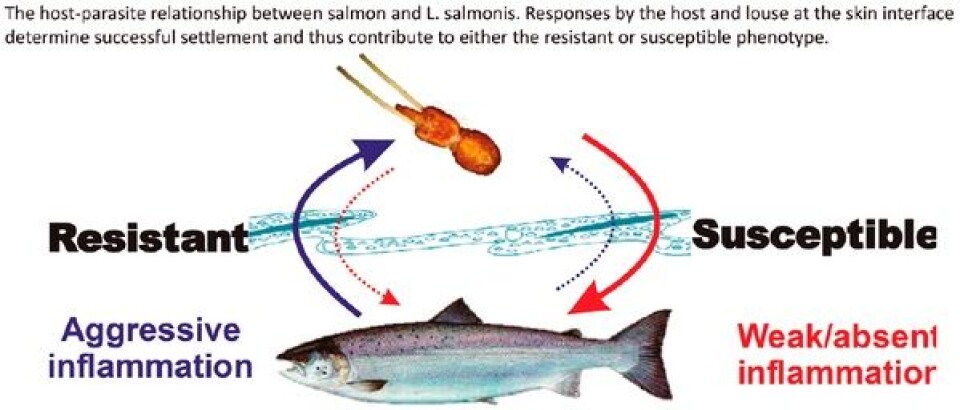
These molecules limit the extent of clotting, increase blood flow, and decrease the host inflammatory responses elicited in an effort to expel the parasite. The actions of bioactive molecules in sea lice secretions appear to affect some species more than others. This may explain the limited inflammatory response at the louse attachment site observed in Atlantic salmon, and the suppressed immune competence of Atlantic salmon towards secondary infections. It is unclear whether the resistant species are able to resist or counter the biological effects of the secretions, or if the lice are not secreting the same complement of molecules when feeding on these hosts. The feeding responses of L. salmonis may depend on the host species consistent with the host preference shown by copepodids during initial settlement. Planktonic copepodids determine host suitability by examining the skin with sensory antennules which is thought to be mediated by factors in the host mucus. Interestingly, coho salmon, which mount a severe inflammatory response to L. salmonis, produce skin mucus that does not stimulate L. salmonis to release the same secretory response compared to the mucus from the susceptible Atlantic salmon. It is possible that resistant host species do not elicit the same secretory components from sea lice as susceptible species. If true, this would likely contribute to the diverse host responses observed among species.
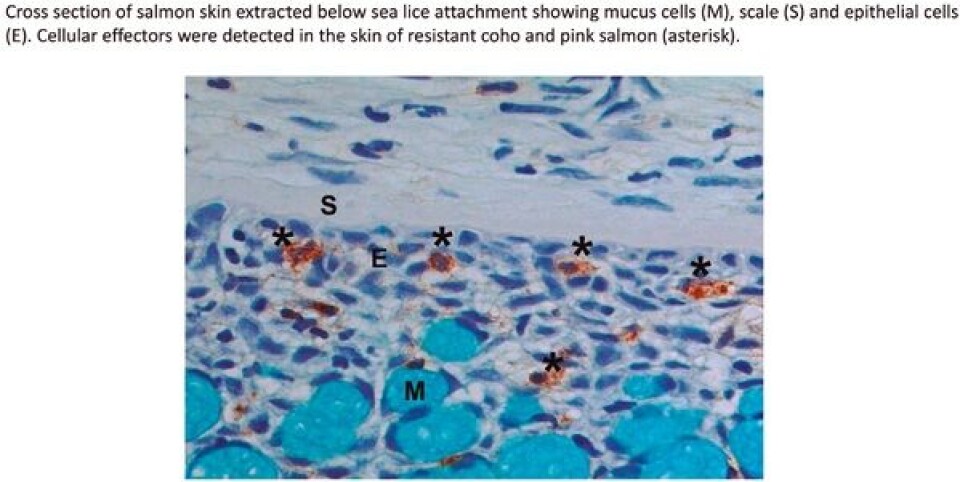
To answer this question I analyzed the feeding response of L. salmonis while parasitizing resistant (coho) or susceptible (Atlantic, sockeye) host species. An exaggerated feeding response was only detected when attached to Atlantic salmon. As the local tissue response of Atlantic salmon to attachment of L. salmonis is characterized by a weak inflammatory reaction and heightened susceptibility, this data presents evidence for an explanatory mechanism. When feeding on Atlantic salmon there is marked expression of virulence factors that act to dampen the inflammatory response at the attachment site. This response by L. salmonis appears to be specific to Salmo spp. as we did not detect similar expression by the susceptible sockeye salmon. Therefore, this work suggests that there may be two independent pathways of susceptibility towards infection with L. salmonis. Firstly, lice feeding on Salmo spp. differ from that of Oncorhynchus spp., in that genetic responses are more robust on Atlantic salmon and characteristic of a more desirable host environment, including enhanced production of energy metabolism, reproduction, and virulence factors. The farmed Atlantic salmon provides a naïve environment that is easier to exploit without an accompanying immune response and has a larger nutritional value-immune response ratio, which would be more beneficial for parasitic exploitation and thus presents a more “tasty fish”. The second pathway of susceptibility describes the variable host response among Oncorhynchus spp., whereby certain species (coho or pink salmon) respond to infection with a more efficient immune response that results in heightened resistance and is a product of variable life histories among the species.
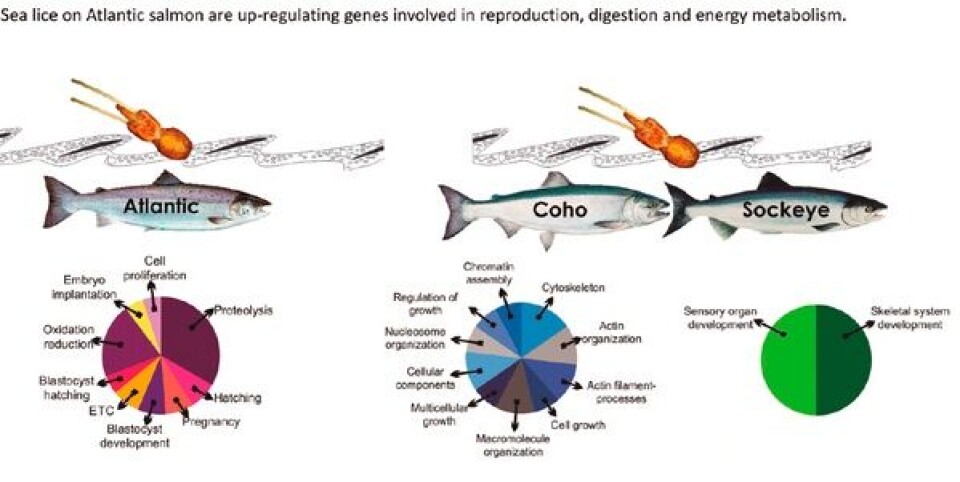
A key piece of the sea lice/salmon puzzle is to determine how species such as pink or coho salmon are able to reject parasite infection. This work has shown that resistance is not limited to host responses, but may be highly influenced by feeding responses (ie secretion of virulence factors) by the parasite. Identifying the species-specific factors contributing to the exaggerated feeding response of L. salmonis while on Atlantic salmon may provide critical information into how to make this host species less desirable to L. salmonis, and perhaps instead “taste” like a coho or pink salmon.

Dr Laura Braden is from a small town in British Columbia. She completed her post-secondary education at a small school on Vancouver Island (Vancouver Island University) and, after working as a research assistant for a professor in the Fisheries & Aquaculture department studying sea lice, she decided to pursue a graduate career investigating this enigmatic parasite. Since starting in a Master’s program in 2009, Dr Braden has secured several prestigious scholarships from the National Science & Engineering Research Council of Canada (NSERC) to study the host-parasite relationship between salmon and sea lice. She has presented her work at over 15 national and international conferences, won international awards for her work, and has been invited to give lectures on her work in labs around the world. Recently Dr Braden was awarded a post-doctoral award from NSERC to continue her research in Prince Edward Island, Canada, working under the supervision of Dr Mark Fast. An avid fly-fisherwoman and aquatic ecosystem enthusiast, Dr Braden can be often found sifting through intertidal zones, pestering octopus at 70 feet, or exploring local rivers to find the perfect spot for an evening cast.























































