
Call made for more research on CRISPR use in salmon
Ragna Heggebø, head of research and development at Grieg Seafood, encourages increased focus on gene editing technology as a tool for precision breeding and improved fish welfare.
During the Tekmar conference in Trondheim, Norway last week, Ragna Heggebø shared experiences from Norway and Canada salmon farmer Grieg Seafood's post-smolt production and R&D activities. In addition, she also highlighted CRISPR technology - a method of gene editing - as an important future tool for precision breeding for salmon.
"CRISPR has the potential to be part of the solution to many of the challenges the aquaculture industry is working on, both on land and at sea," she told Fish Farming Expert's Norwegian sister site, Kyst.no.
'Norway should be a leader'
CRISPR technology was also a topic in a panel debate where, among others, State Secretary Even Sagebakken participated. Here, Heggebø advocated for more research and development in gene editing as part of the future of aquaculture.
"We need political facilitation for cautious testing of CRISPR technology. This must happen in safe environments before we consider a larger scale," she stated during the debate.
Heggebø emphasised the importance of positioning Norway as a technological leader in aquaculture.
"Norway should be a pioneer in technology development. If we do not facilitate now, we risk falling behind countries that are already using this technology."
CRISPR-sterilised salmon
Heggebø told Kyst.no that she believes the release of experimental fish made sterile by use of CRISPR technology is a necessary first step to test this technology in controlled environments.
"Sterile fish ensure that wild salmon are not affected if farmed fish were to escape. When the fish are sterile, they also do not need to go up the rivers to spawn," she pointed out.
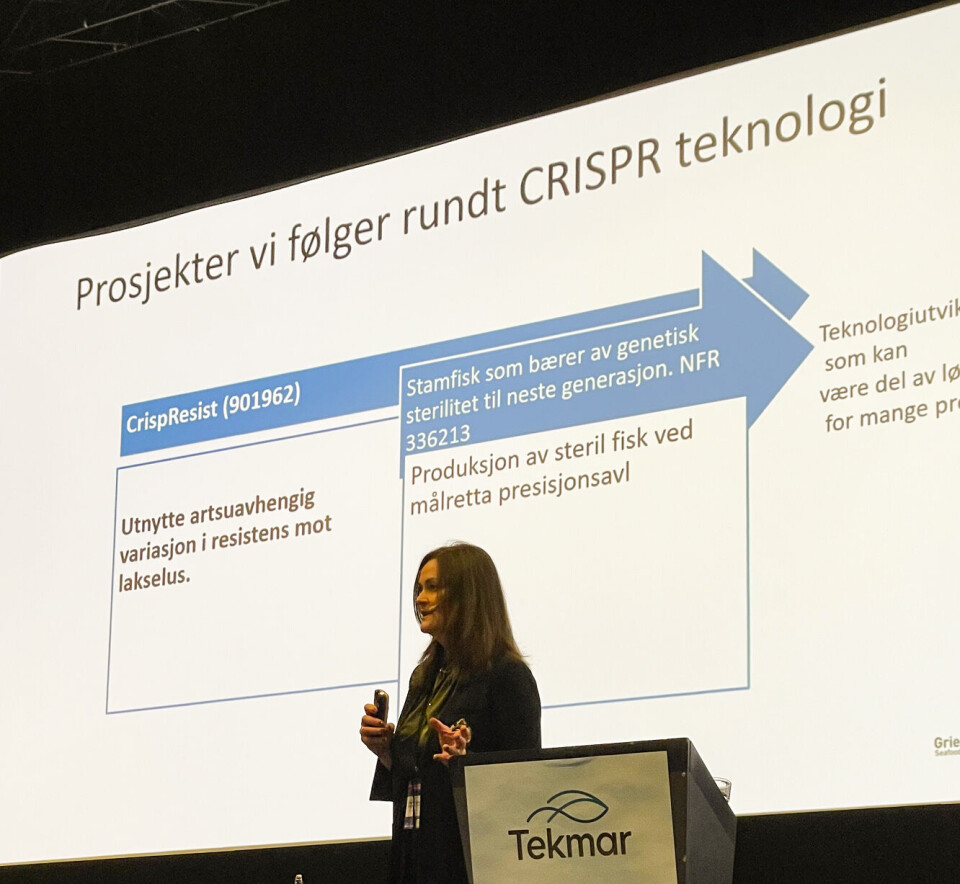
Today, CRISPR research in Norway is limited to laboratory experiments. The Institute of Marine Research has applied to release sterile fish in Matre, but the application was rejected. Read more about this here.
Solutions to major challenges
Heggebø points to several challenges that CRISPR can help solve, including production with sterile farmed salmon and utilising naturally occurring genetic variation in resistance to salmon lice.
"The impact farmed salmon has on wild salmon stocks is taken very seriously. We need more alternatives to the current method of sterilising salmon, which is triploidisation of eggs," she said.
The R&D manager also emphasises the salmon lice issue, which has been a major challenge for the industry.
"This is complex and takes time. CRISPR gives us the opportunity to streamline breeding work and strengthen the salmon's resistance to sea lice," said Heggebø.
Support for regulatory changes
Grieg Seafood submitted its response earlier this year to the Biotechnology Committee's report NOU 2023:18, expressing its support for an update of the regulations for gene editing, allowing for a distinction between traditional GMO (genetically modified organisms in which foreign genetic material has been added) and gene editing (like CRISPR).
"We support the majority's proposal to modernise the regulation of organisms developed with techniques with great potential, such as CRISPR. This can give us the tools we need to solve challenges in a sustainable way," said Heggebø.
The R&D manager concluded with an appeal to the politicians: "We must dare to invest in the development of new technology. This has the potential to be safe, cost-effective, and is already in use in many other countries. Norway should seize this opportunity to ensure that we keep up with global developments."
Grieg Seafood already uses sterile salmon in Placentia Bay, Newfoundland, Canada, as part of its licence conditions. These are made sterile by pressurising ova, a technique which changes the fish from diploid (two sets of chromosomes) to triploid (three sets of chromosomes). Research has shown that triploid fish can be less robust than diploids, although Grieg Seafood NL says it has not had any issues linked to triploidy.



































































