Next-generation vaccine required to beat SRS, says Pathovet chief
The strategy to develop effective vaccines against the bacterium Piscirickettsia salmonis, which causes salmon rickettsial septicaemia (SRS) must change, according to the chief executive of Chile-based international fish health services company Pathovet.
In a detailed article, Dr Marco Rozas reveals all the advances in the study of the bacterium and what is missing to achieve, in part, its control, such as the lack of efficacy of vaccines.
SRS, also known as piscirickettsiosis, is a significant cause of antibiotic use in salmon farming in Chile and Canada and has also occasionally occurred in Scotland and Ireland.

‘Know the enemy’
In his paper, Rozas asks how the ever-present risk of negative consequences of antibiotic use in salmon farming can be balanced with the productive and economic viability of an animal production industry, as well as care for the aquatic environment and public health and sustainability.
In the paper, which reviews current knowledge of P. salmonis, Rozas writes that “we must know the enemy and how it interacts with its host. A lot of knowledge has been generated using this line of research, however, it remains insufficient”.
Evasion of immune response
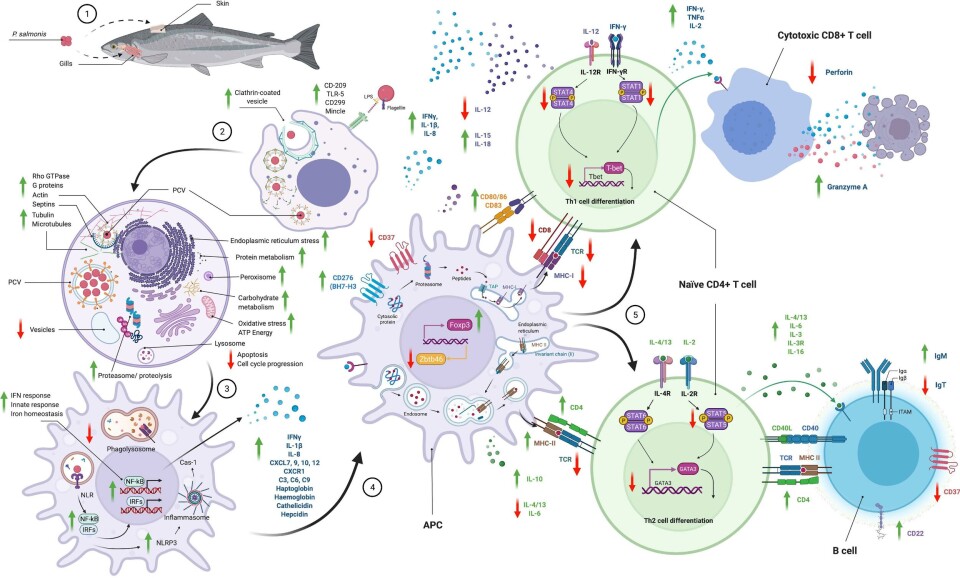
The review ranges from aspects of the biology and pathogenesis of the bacteria to the evasion of the immune response and failure of current vaccines, passing through the complex mechanisms of interaction between the bacteria and the fish.
Among the highlights of the paper, it is explained how, unlike viruses, P. salmonis can effectively evade the adaptive immune response mediated by fish cells, which Rozas defines as a “checkmate”.
“When the bacterium is alive and infects fish, it is plausible that virulence factors support its strategy to escape antigen processing and/or that MHC-I does not present antigens efficiently, and the bacterium eventually manages to evade the response mechanisms of the fish,” he writes.
The next question Rozas raises is whether this is the reason why the fish are also unable to activate a response against a bacterin-like vaccine or even a live attenuated vaccine.
Faced with this, Rozas argues that basically, the strategy to develop effective vaccines against P. salmonis must change, starting by understanding the biological pathways used by the pathogen to infect and replicate in host cells, and then defining strategies to block them.
“The conventional approach used historically for the development of vaccines against P. salmonis has not been the solution to the problem, so salmon farmers have no choice in the field against SRS outbreaks other than to use the last card they have available, drugs”, he says.
Reverse vaccinology
Faced with such a problem, “the time has come for new generation vaccines and reverse vaccinology in aquaculture: DNA, mRNA, viral vectors, chimeric multiepitope and multiantigenic vaccines, among others,” he says.
“However, it is necessary to generate deeper and more specific knowledge about the interaction between P. salmonis and fish cells and, of course, generate the corresponding regulatory bodies for the formal registration of these new vaccines. It is not prudent to develop new generation vaccines without a regulatory structure that authorises and regulates their commercial use in advance.”
The open access paper, Why Does Piscirickettsia salmonis Break the Immunological Paradigm in Farmed Salmon? Biological Context to Understand the Relative Control of Piscirickettsiosis, is published in the journal Frontiers in Immunology and can be read here.