How much Listeria can be in fresh salmon for it to be eaten raw?
By Elin Reitehaug and Taran Skjerdal, National Veterinay Institute

An increasing part of the population, both in Norway and the rest of the world, consumes salmon in the raw state rather than to heat treat it before consumption, and one can ask the question: should salmon be considered ready to eat food? And in this case, should one distinguish between whole salmon, fillet, fillet pieces or finished products - or is the fishing industry in agreement that all fresh salmon sent to the market can be eaten raw? Should one possibly distinguish between fish that is meant to be consumed raw and fish that might inadvertently be considered ready to eat? Sushi and smoked salmon are typical products that are sold in large quantities, where the fish is not intended to be heat treated before consumption. “Most people” now consider raw salmon as safe food, which leads to stricter requirements for manufacturers to supply fish of a quality that can be consumed raw.
It is no secret that many manufacturing companies are struggling with Listeria in the production environment. Listeria is actually a whole family of bacteria, of which Listeria monocytogenes can cause serious illness in humans. Often people in risk groups become ill from Listeria, especially the elderly, people with compromised immune systems and pregnant women (fetus). Even a low number of bacteria, right down to 100 colony forming units (cfu) per gram, can cause disease in at-risk groups, and an infection can be fatal and cause abortion in pregnant women. Healthy people will not usually get sick from these bacteria, but high doses can result in mild symptoms that are usually harmless. In 2012, 31 cases of Listeriosis were recorded in Norway. This is about as frequently as in the rest of Europe, when measured per number of inhabitants.
It may seem as if there is unnecessarily strong focus on listeriosis when there are so few cases of illness, but Listeria is considered a food safety issue for several reasons. The number of cases of listeriosis has increased in Europe over the past decade. Listeria is found virtually everywhere - bacteria are found naturally in water, soil, plants and animals. Listeria in food can come from both raw materials, from infection by humans through contact or from production equipment. Listeria can grow at refrigeration temperatures, both with and without access to oxygen, and it can tolerate high concentrations of salt. Moreover, Listeria can get established in manufacturing companies in places where it is difficult to keep clean, in tears and links or biofilm, and it can be very difficult to get rid of the bacteria once it is established. One must therefore expect to find Listeria in food from time to time, even with good production hygiene. However, it is possible to keep the number of Listeria in food so low that it does not cause disease. This can be done by keeping the temperature low, reducing shelf life, adding preservatives, labeling the food to be properly heat treated before use, or other measures. There is thus a considerable risk of catching listeriosis if one ignores that Listeria can be present in food, but quite possible to limit if one takes the risk seriously and takes risk-reducing measures.
The consequences of listeriosis are very serious, at worst it can cause death, and social costs are very high. A case of listeriosis is calculated to be about 500 times more expensive (for society) than a case of campylobacteriosis. The combination of the facts that listeriosis is a very serious disease for humans, that Listeria in food occurs relatively frequently, and that Listeria in food can be kept below disease causing numbers with relatively simple means, causes a strong focus on Listeria monocytogenes in legislation and with customers, including buyers of Norwegian fish. The legislation requires manufacturers of ready-to-eat food to risk-assess their products with regard to Listeria. European guidelines on how this can be done, have been developed. Te reference laboratory for Listeria in Europe (ANSES) has led the work, and the Veterinary Institute has participated in the working group. The guidelines have been tried for salmon and sushi in the Baseline project. This means that the results presented here can be used as evidence that risk assessment of these products has been done.
Guidelines and criteria for Listeria The rules for monitoring and control of Listeria in ready-to-eat products have become stricter. This is because there has been an increase in Listeria cases in Europe over the past decade, and that there are constantly new “ready-to-eat” and “ready-to-heat” products on the market. The current legislation for Listeria in ready-to-eat food is the Microbiological Criteria No. 2073/2005 of the EU. In the regulations, the authorities lay down the overriding criteria, and it is up to the food companies to “translate” these into the best practices for their own production. It is also up to the manufacturer to decide if the product shall be considered ready to eat food or not.
The new legislation focuses on risk assessment, taking into account the entire food chain from farm to fork. The new mindset also takes into account aspects other than food safety around food production – such as to minimize the amount of food thrown away, food safety, quality and economy. It is not realistic to think we can guarantee one hundred percent safe food, and the regulations provide procedures to risk-assess food production so as to achieve food that is safe enough. For Listeria the regulations require there to be below 100 cfu per gram of product on the last day of the product’s shelf life, or not detected when dispatching the product, both based on the analysis results from at least five samples.
The risk assessment should thus be based on an adequate level of protection (alop). When this is set, other criteria (food safety objectives, FSO) are set to be used in the process to ensure that this is maintained, and this coincides generally with the limit on the last day of the shelf life for the specific microbe (for Listeria this is 100 cfu per gram of sample material). Thereafter one defines one’s goals (performance objectives, PO) and performance criteria (performance criteria, PC) in the early stages of the manufacturing process, which makes it possible to check that these criteria are met. To do this one needs both good analysis methodology and knowledge of how the bacteria grow in the product one wants to risk-assess. The problem with Listeria is that they grow in cold storage, and they have different growth rates in different products. Then the challenge is to strike a balance between ensuring safe enough fish for the market and putting too stringent shelf life requirements which may result in financial losses and larger amounts of food waste.
Current analytical methods for Listeria Listeria can be analyzed quantitatively or qualitatively. In a quantitative analysis 25 grams of sample will be analyzed and this will give the of number of Listeria per gram sample. The problem with today’s quantitative assays is that one has a detection limit of 10 cfu per gram. One will thus be able to conclude that the product has less than 10 cfu Listeria per gram, but you will not know if there are 5 or none at all. A qualitative analysis includes a step in which Listeria receive optimum conditions to multiply, and one will then find out whether there are Listeria in the sample or not. However, one will not know if there were 5 or 1 000 bacteria present before amplification. Usually a combination of these two methods is used. When it comes to the problem of Listeria in fresh salmon, a combination of these two methods will not be good enough, since it is not necessarily required that the fish must be completely free of Listeria to be regarded as safe. There may also be considerable variation within a product batch, and the samples taken are not necessarily representative of the whole batch.

Baseline - to find solutions around sampling and analysis of Listeria in food Problems of sampling and analysis of food are examined in the EU project Baseline - “Selection and improving of fit-for-purpose sampling procedures for specific foods and risks”. The National Veterinary Institute (NVI) has led the section on seafood, and together with partners from Spain, Croatia, Italy, France and Norway examined Listeria in fresh salmon, smoked salmon and sushi, toxins from Vibrio and viruses in shellfish and mercury in tuna.
A large number of samples of fresh salmon have been examined at NVI. Two types of samples were examined: those that were naturally infected from a facility that had challenges with Listeria, and samples of fresh salmon from a store where Listeria was added in the laboratory to create models and “worst-case” scenarios. The surveys included both sampling procedures and alternative ways of taking samples, new and improved methods of analysis and modeling to calculate how much Listeria may be permitted in salmon on the production date for it to still be considered safe enough and contain less than 100 cfu Listeria per gram at last use date.
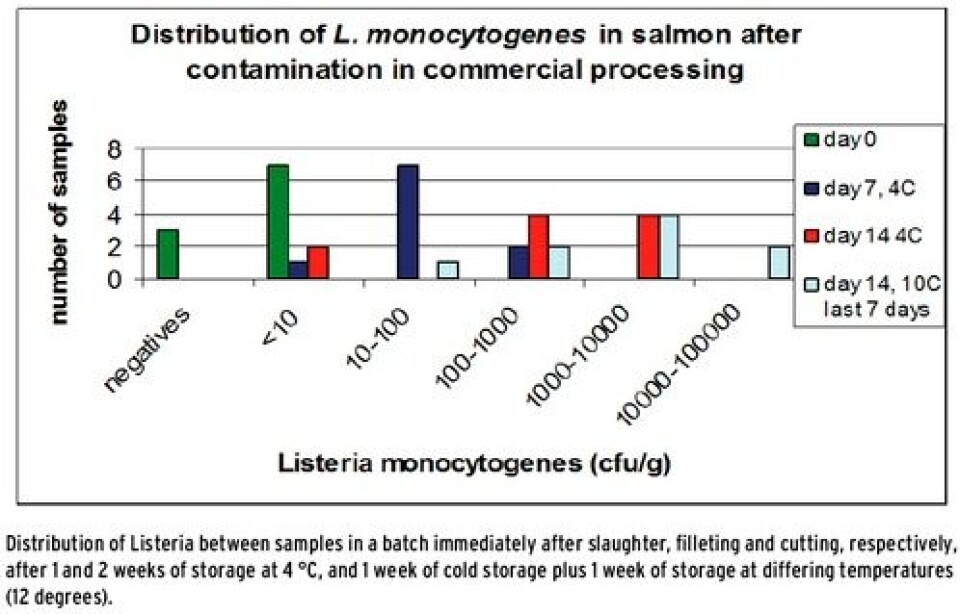
Growth of Listeria in naturally contaminated samples of salmon Figure 1 shows the distribution of Listeria in a batch with naturally contaminated samples of salmon. Samples were sent frozen straight from the production, and were thawed in a refrigerator and stored at 4 °C for up to two weeks. Ten of the samples were moved and stored at 10 degrees during the last week to promote growth. Ten samples were analyzed from each sampling (days 0, 7 and 14) in order to detect the variation in the batches. The results showed that Listeria grows in naturally contaminated samples at 4 degrees. It was also shown that there is an extreme variation within the batch, and that there are high concentrations of Listeria in several samples after cold storage - despite the fact that none of the ten samples from the first sampling day contained more than 10 cfu Listeria per gram sample. The analysis was repeated for eight different batches during the course of a year with the same result. How can manufacturers deal with samples of salmon that are apparently negative at the date of production, but with values far above the threshold value after one and two weeks’ storage?

How should one take samples of raw salmon? When manufacturers take samples during manufacture and distribution of products, is it possible for them to relate to threshold values days or weeks ahead of time? Yes, it is! It may be possible to detect Listeria early in the process even if the numbers are low, but this requires that one has a good sampling regime that provides a representative picture, and that one uses methods that are more sensitive than those commonly used today.
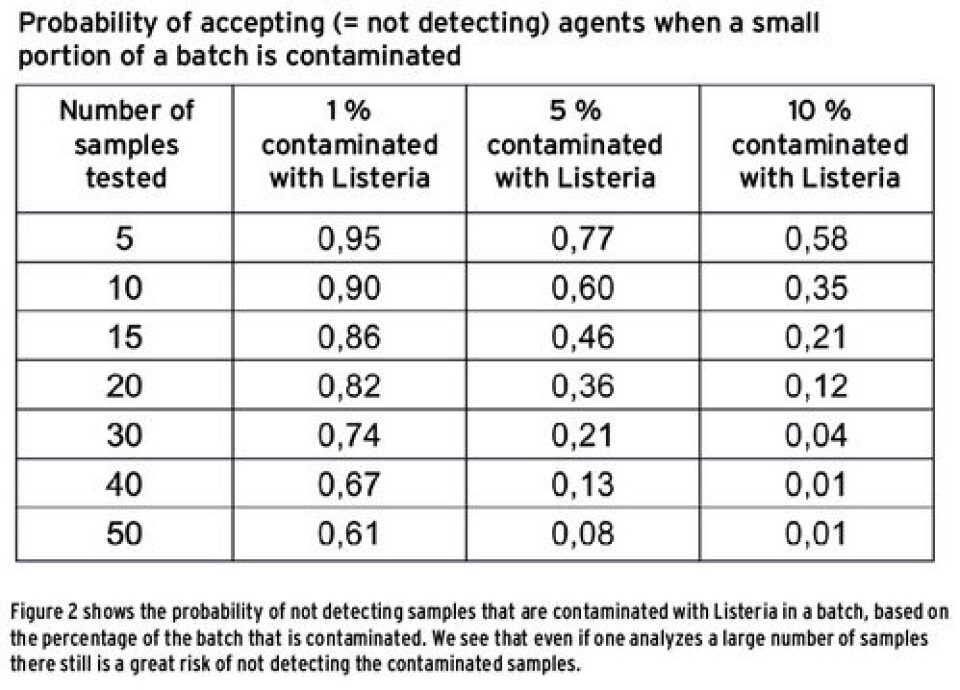
From naturally contaminated samples of salmon, we found that the bacterium can be very unevenly distributed in a batch. This means that one must either take many samples to detect those that are contaminated or that one has to find other ways to take samples that can give a representative picture of a batch. If only a few samples are contaminated, there is little chance that these are picked out and tested, and the whole batch will then be sent out on the market, including the contaminated samples. Figure 2 shows the chance of not detecting Listeria in a batch if only a small portion of the samples are contaminated. From the figure we see that if one of 100 samples is contaminated, there is 61 % chance of not detecting this even if one analyzes half of the samples. If 10 out of 100 samples are contaminated with Listeria, there is still 1% chance that one will not detect bacteria if one analyzes half of the batch (50 samples). It is far from realistic that one should analyze so many samples! How can one take samples in the best possible way to safeguard against a false negative result? The safest method is of course to keep the production facilities free of Listeria and take frequent samples from the environment such as conveyors, storage tanks, cutting machines, gloves, washing water and drains. We also examined how Listeria spreads in pieces of raw salmon by stacking cubes of raw salmon in height and width, and adding Listeria to one of the pieces. We found that Listeria is moving outwards and downwards in the fish. This indicates that instead of taking samples of whole salmon from the top layer of a crate, one should rather take the fish from the bottom of a crate for analysis. One can also consider taking samples of runoff water in the bottom of a crate, which will give a result for a whole lot of samples. If the existence of Listeria is confirmed in the production environment or in the runoff water, it is likely that the product is contaminated to a greater or lesser extent.
Modelling of growth curves If the growth potential of bacteria in a product is known, one can estimate a limit for the product at an early stage based on the limit value for the product at the date of expiry. In this way one can determine the amount of Listeria that may be permitted at an early stage for fresh fish. Since this may represent a very low number, even below 1 Listeria per gram of fish, this will require more sensitive methods than those traditionally used. It is also important for growth studies to use realistic temperature scenarios, which should take into account the processing of fish both during production, during transport, in shops and homes of consumers.
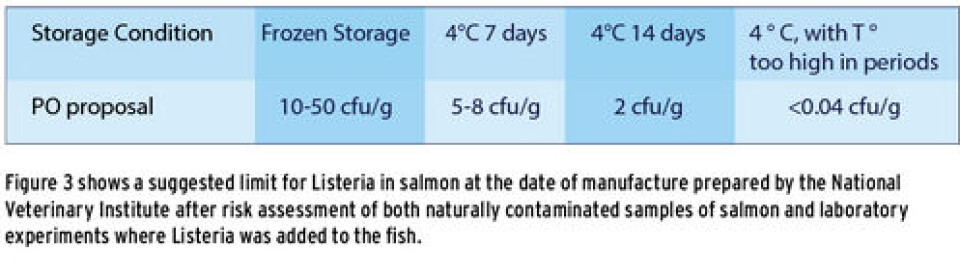
Figure 3 shows a suggested limit for Listeria in salmon at the date of manufacture, prepared by the National Veterinary Institute after risk assessment of both naturally contaminated samples of salmon and laboratory experiments where Listeria was added to fish. From the figure we see that if one is to guarantee that raw salmon contains less than 100 cfu per gram salmon at the date of expiry, then the fish must contain less than 5-8 cfu Listeria per gram sample at the date of production if the shelf life is to be 7 days and less than 2 cfu per gram sample at the date of production if the shelf life is to be 14 days. In order to detect this one needs more sensitive analytical methods than those commonly used today.
More sensitive methods of analysis In order to detect down to 2 cfu Listeria per gram sample we looked at various methods that can reduce the detection limit. A very simple method that is based on current methodology from NMKL and ISO is simply to reduce the amount of diluent in the sample material. Typically, a sample gets diluted 10 times in a solvent before it is spread on agar plates to count the number of colony forming units. One will then be able to detect down to 10 cfu per gram Listeria, and if one does not get growth on agar plates, one can conclude that there are less than 10 Listeria per gram sample. Just by diluting the sample volume twice with solvent, one can lower the detection limit down to 2 cfu per gram. 1 ml gets spread on three agar plates, and one can count down to 2 colony forming units per gram of sample. This method has been tested on a number of samples in the laboratory, both naturally contaminated samples and samples with added Listeria.
We have also developed methods that can quantify down to 1 Listeria per 5 grams of fish. The method is designed so that samples can be taken and sealed in plants to avoid Listeria from growing in the sample during transport from the time the sample is taken until it reaches the laboratory. During regular sampling and submission to the lab Listeria can grow and the number measured will be higher than it really is in a sample. The method is not quite fully developed yet, but a prototype is now being tested at a plant. The method is also planned to be tested in three other plants throughout autumn. If the trial is successful, the method can be available in a few years time.
The big question with Listeria in food is how high a number of bacteria is acceptable without compromising food safety, legislation and other customer requirements. Studies performed by Baseline show that traces of Listeria in fresh salmon can be accepted, even if the fish is to be used for sushi or smoked salmon. If the fish is to be stored chilled, the number must be below 2-8 grams per fish, depending on how long the fish is to be stored. Since Listeria is often unevenly distributed in the batch, seven samples of the fish should be taken, preferably from the bottom of bins or other vessels to document that the number is as low. The 7 samples may in some cases be analyzed as pooled samples. Standard analytical methods today are not sensitive enough for the results to be used directly, but we have developed more sensitive methods, so it should not take too long before they can be used.
These studies have been possible because we have been able to analyze samples from a producer who has had Listeria in his plant for several years, but has managed to keep the number low enough so that food safety has not been compromised, as far as we know. We therefore assess the prevalence and numbers of Listeria as low as one can realistically expect when Listeria is established in a plant. In our view, the occurrence and number of Listeria in other enterprises can be assessed against our data with regard to how low the number of Listeria should be for fresh salmon to be considered “safe enough” to be eaten raw.























































