Amoebic Gill Disease
By Dr. Hamish Rodger, Vet-Aqua Internationalwww.vetaquainter.com
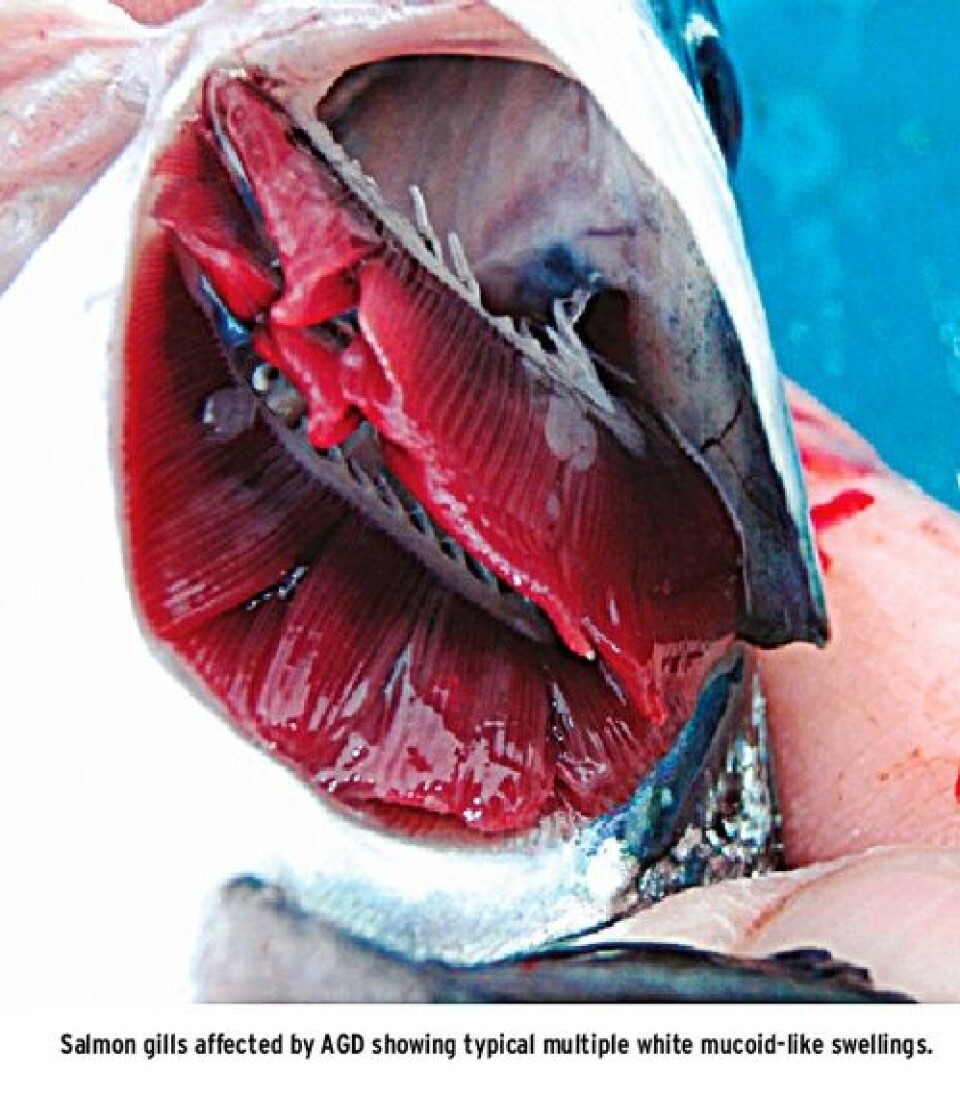
What is AGD? Amoebic gill disease (AGD) is a condition of marine farmed fish, mainly salmonids, which causes gill pathology. It results in ill thrift, poor performance and mortalities. In marine salmon farming it is caused by an amoeba named Neoparamoeba perurans. This marine amoeba is free living and parasitic and when it colonises gill tissue can cause a dramatic cellular reaction in the fish which results in proliferation and fusion of the gill anatomy. This appears as pale grey or white, mucoid-like swellings on the gills which as they progress affect more and more of the gill surface. These changes in the gills lead to compromised or partial failure of the normal gill functions including respiration (uptake of oxygen and discharge of carbon dioxide), osmoregulation (control of salts and fluids in the body), control of acid/base balance and excretion of nitrogenous waste from the body (mainly as ammonia). There is also evidence that the gills have a role in monitoring both blood oxygen and carbon dioxide through sensory neurons (Evans et al. 2005). The impact of compromised gills on fish health will therefore be profound, and in the case of AGD this gives rise to fish that lose the ability to feed, have difficulty breathing properly and also lack normal control of salts, fluids and blood gases and as a result if left untreated will die. More subtle gill changes may make the fish more vulnerable during periods of low oxygen or stress such as when water temperatures are high, when fish are crowded for sea lice bath treatments or when transported for harvest. There are also freshwater amoeba species which can cause problems for trout farms and other species, however, the focus for this article are those of marine origin.
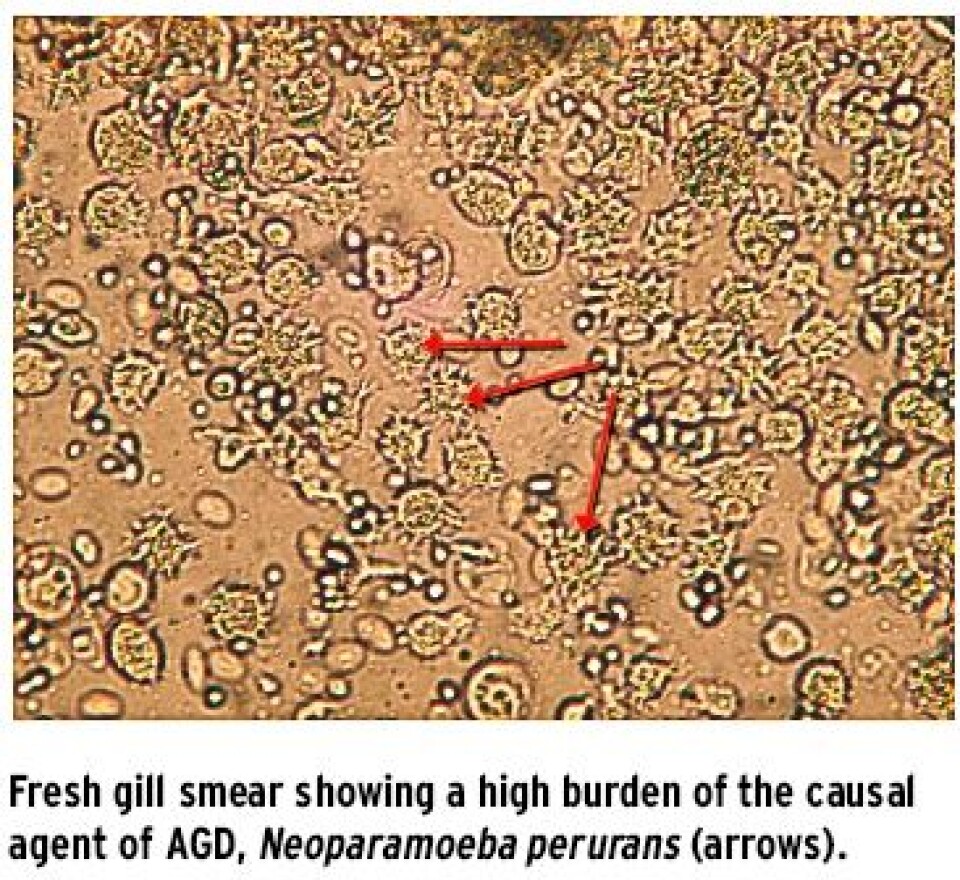
Who has it? In the salmon farming sectors the initial reports were from Tasmania when shortly after farming started there in the mid-1980s AGD was first recognised and it continues to be a challenge to this day, with well-established control methods in place to manage this condition. AGD has been recognised in most marine salmonid farming regions including the USA (Washington), Chile, France, Ireland, Scotland, Spain and Norway. Other fish species affected by AGD include farmed turbot (Psetta maxima), sea bass (Dicentrarchus labrax) and ayu (Plecoglossus altivelis) in Spain, France, Portugal and Japan (Mitchell & Rodger 2011).
Where has it come from? Marine amoeba are ubiquitous and have been described in the marine environment as free living and also as parasitic organisms from many species including urchins, crabs and lobsters. The causal agent of AGD, N. perurans, does appear to have global distribution and has been detected by molecular techniques (PCR) in farmed salmon and turbot. Samples taken by Vet-Aqua International have also been screened by PCR and have detected N. perurans in sea lice (L. salmonis), the biofouling hydroids (E. larynx) and mackerel (S. scombrus) in pens with AGD affected salmon. However, there remains a large gap in the knowledge about the biology of this parasite. Results of studies in Australia have shown that densities of the amoeba are significantly higher in the summer and that bacterial counts, water turbidity and temperature are significantly associated with amoeba densities (Douglas-Helders et al. 2003).
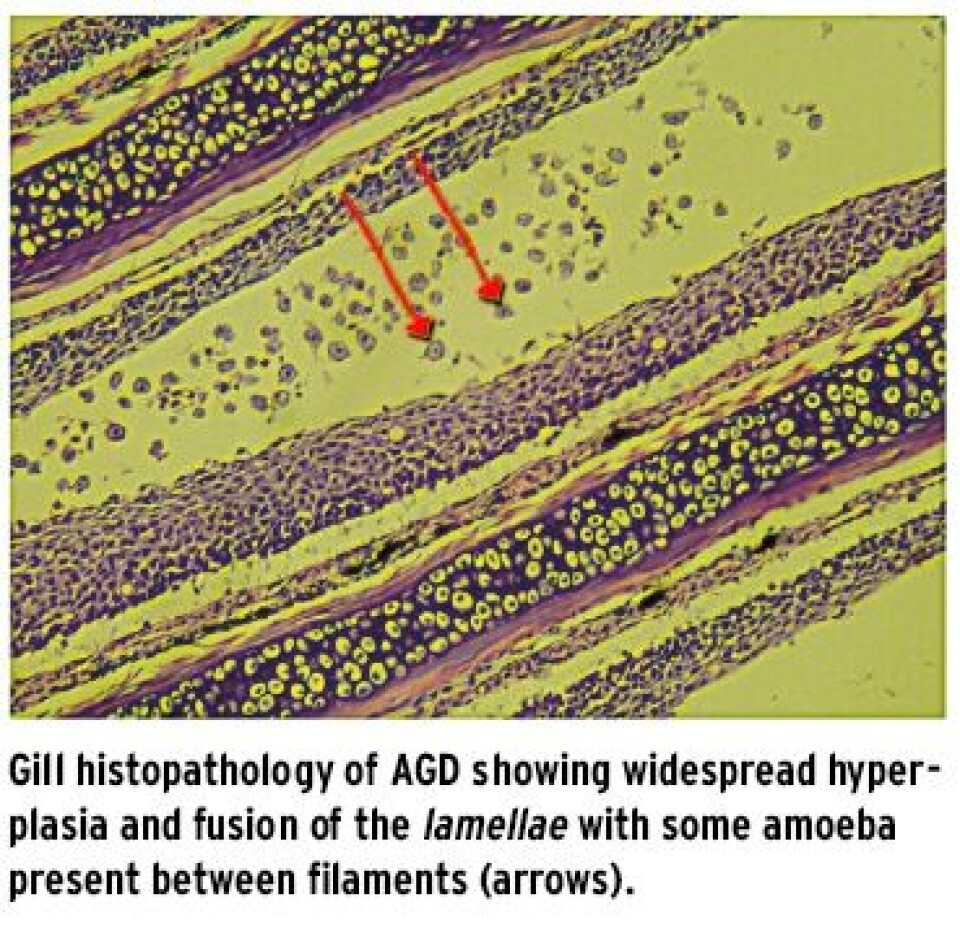
When does it cause problems? The parasite elicts a dramatic response in farmed salmon gills and the numbers of amoeba and response in the gills appears to be affected by various aspects such as original gill condition of the fish, presence of other disease, genetics, age (or size), water temperature and water salinity with the problem most common during periods or sites with high, oceanic salinity. Farm sites with freshwater flushes or that are brackish are rarely affected. It appears that the parasite can be dormant at low temperatures but when temperatures rise there can be a rapid increase in the amoeba and as oxygen is also less available this makes for a more challenging rearing environment. Some research has indicated that fish may not actually succumb from respiratory failure, however, with the main challenge being associated with acid-base disturbances with the increased mucus on the gills impairing carbon dioxide excretion from the gills. Cardiac dysfunction has also been described in AGD affected salmon (Powell et al. 2008). In some cases of AGD focal liver pathology is also sometimes observed.
Why has it emerged as a challenge? This question remains to be robustly answered and there are many theories as to why the amoebae have emerged as a challenge. Environmental conditions for the amoebae may have changed to allow for more rapid multiplication, the amoebae themselves may have changed, or the rearing conditions may have developed to allow for more suitable substrates for the organisms, an increase in predisposing gill conditions may allow easier colonisation and/or it could be a combination of some (or all) of these factors. One thing we do know, however, is that the issue has now emerged and it may continue to be with us for some time.

How can it be controlled? Good biosecurity and husbandry will help reduce the risk of contracting the condition and fallowing, single generation year class sites, avoiding moving infected fish and rapid removal of any mortalities will all help in the control of all infectious disease and apply equally to AGD. The amoeba can survive in seawater and may, it has been suggested, also be spread by wild vectors so there is a degree of risk if your site is high salinity and in a region where other cases have been confirmed. Regular monitoring of gill condition for the typical pale grey/white spots on a weekly basis (as when doing routine lice counts) and scoring and recording of the gills for any spots (score 0 [no spots, gills healthy red colour] to 5 [extensive lesions covering most of the gill surface]) will also be important and if any suspect lesions are observed these can then be checked with fresh microscopy, histology and/or PCR. Early intervention and treatment can then in instituted and in certain areas of Tasmania the fish are routinely bathed in freshwater for 2 – 3 hours for up to 12 to 15 times through their seawater on-growing phase. This adds significantly to the cost of production, however, in Tasmania now there are very little stock losses from AGD. The lack of availability of freshwater in Scotland and Ireland and the absence of an established infrastructure to use freshwater has meant that some sites have trialled hydrogen peroxide as an alternate therapy and although there are risks with this treatment it has proven effective in reducing the amoeba burden or clearing infection in some cases. The variables that should be taken into account with a peroxide treatment include gill condition, water temperature, organic loading in the water, size of fish, whether treating in a well-boat or tarpaulin, etc.. There is no effective vaccine for the disease despite many years of research, however, fish do appear to develop immunity to the condition and there is evidence from Australia which indicates that there is genetic variation to AGD pathology and survival in Atlantic salmon (Taylor et al. 2009). Training of farm staff in how to screen for, sample and examine fish for AGD is also important as early detection and action will ensure that the impact from this disease can be minimised. Further details on in-house or on-site training can be obtained via vetaquainter@gmail.com References Douglas-Helders et al. (2003) Journal of Fish Diseases, 26, 231 - 240 Evans et al. (2005) Physiological Reviews, 85, 97 - 177 Mitchell & Rodger (2011) Journal of Fish Diseases, 34, 411 – 432 Powell et al. (2008) Journal of Fish Biology, 73, 2161 – 2183 Taylor et al. (2009) Aquaculture, 294, 172 – 179























































